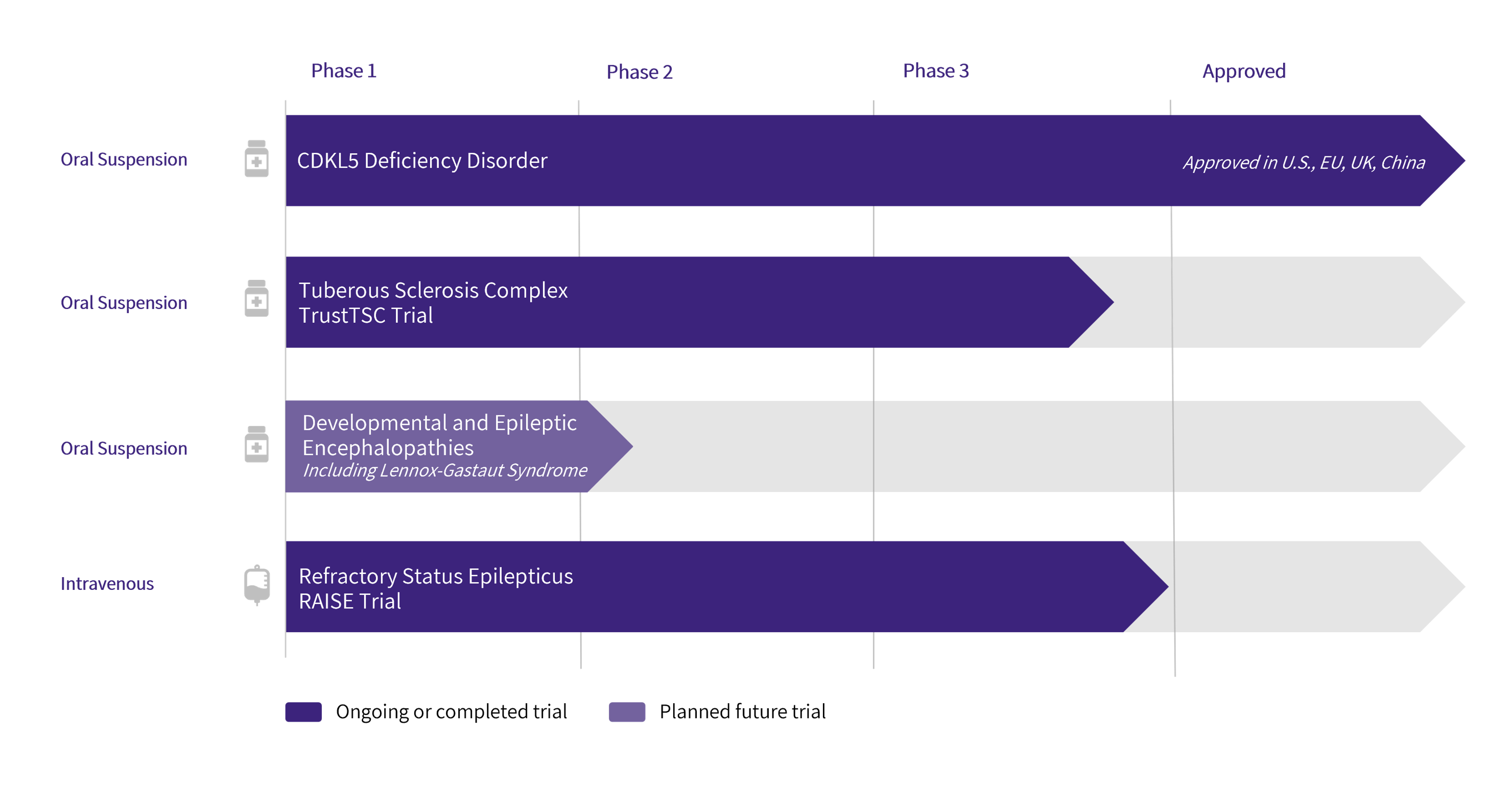
Pipeline
Clinical Trial Information
Ganaxolone is being developed in intravenous (IV) and oral formulations intended to maximize therapeutic reach to patient populations in both acute and chronic care settings.
Information about Marinus’ clinical trials can be found on ClinicalTrials.gov.